Austin, TX, USA, July 30, 2025 (GLOBE NEWSWIRE) — Custom Market Insights has published a new research report titled “Lenacapavir Injection Market Size, Trends and Insights By Indication (HIV Treatment, Pre-Exposure Prophylaxis (PrEP)), By Formulation (Injectable, Oral Tablets), By Distribution Channel (Branded Medicine, Generic Medicine), and By Region – Global Industry Overview, Statistical Data, Competitive Analysis, Share, Outlook, and Forecast 2025–2034“ in its research database.
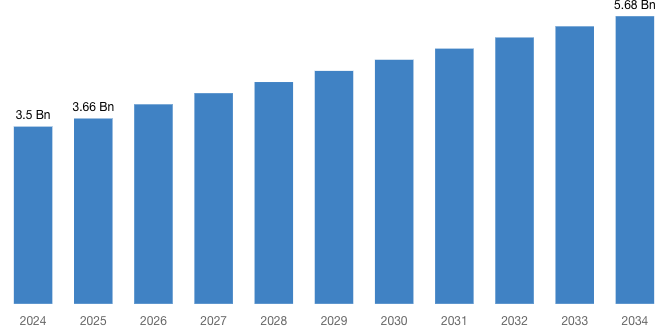
“According to the latest research study, the demand of the global Lenacapavir Injection Market size & share was valued at approximately USD 3.5 Billion in 2024 and is expected to reach USD 3.66 Billion in 2025 and is expected to reach a value of around USD 5.68 Billion by 2034, at a compound annual growth rate (CAGR) of about 5.1% during the forecast period 2025 to 2034.”
Click Here to Access a Free Sample Report of the Global Lenacapavir Injection Market @ https://www.custommarketinsights.com/request-for-free-sample/?reportid=71836
Overview
As per the industry experts at CMI, Market participants, such as Gilead Sciences, are concentrating on ultra-long-acting antiretroviral therapies for HIV treatment adherence challenges, with Gilead Sciences focusing on Lenacapavir Injection market. Innovations focus on subcutaneous delivery systems, dosing intervals, and pharmacokinetics. Collaborations with public health and clinical organizations are propelling international approvals and access.
There are further combination regimens and formulation innovations targeted at HIV with multidrug resistance. Market expansion occurs in low-income countries with equitable pricing, reinforcing social responsibility and supporting ESG objectives. Responsibly managed corporations fill the gaps for global therapy and preventive health care.
Key Trends & Drivers
- Long-Acting HIV Treatment Demand: The treatment lenacapavir, which is administered biannually, is a game changer for patients who have difficulty adhering to daily oral regimens. Lenacapavir’s long acting form decreases adherence-related complications and mitigates pill fatigue while improving overall patient convenience especially in low resource settings. Health systems are adopting long-acting injectables as part of simplified HIV care models. This is most notable in high burden areas where providers seek long-term treatments that preserve viral load suppression during periods of infrequent monitoring. This shifts convenience to the provider, which is currently driving adoption globally.
- Rising Prevalence of Multidrug-Resistant HIV: The clinical challenge posed by an increasing population of patients suffering from multi-drug resistant HIV infections is effectively met by lenacapavir. Lenacapavir, unlike other traditional antiretroviral therapies, maintains its position as a lifeline for individuals requiring extensive treatment history due to its activity against a wide range of HIV strains. Such resilience increases its applicability for salvage therapy regimens. Its novel action as a capsid inhibitor enables it to plug therapeutic holes left by other drugs. Hence, cases of MDR HIV, particularly in Sub-Saharan Africa and Eastern Europe, are increasing and lenacapavir is demanded as a critical component of resistance management.
Request a Customized Copy of the Lenacapavir Injection Market Report @ https://www.custommarketinsights.com/request-for-customization/?reportid=71836
- Regulatory Support and Global Access Programs: Lenacapavir is approved and awarded incentives by the FDA, EMA, and Health Canada as a treatment for HIV due to its unique features and promise. These approvals have expedited regional access. At the same time, Gilead collaborates with PEPFAR, WHO, and the Global Fund to incorporate Lenacapavir into their treatment programs for middle and lower-income countries. Such collaborations enhance access and coverage especially in regions highly affected by HIV. The combination of these actions—regulatory, humanitarian, and commerce—strengthens the global supply strategy for lenacapavir while keeping the commercial strategy seamless.
- Technological Advancements in Injectable Formulations: New technologies that enable extended release of therapeutics through injections have made it possible for Lenacapavir to maintain therapeutic levels for longer periods. Advances in administering injectable therapeutics have made them easier to submit to put to use in almost all healthcare settings, such as initiated bioavailable, lower Mentors devices, and even autoprinters. These improvements also lessen the workload placed on health facilities. They offer discreet and effective treatment to patients. Reduced clinic appointment attendance caters to both rural and urban areas, improving the healthcare system as a whole. The evolution of injectable technologies strengthens Lenacapavir’s formulation strategy, resulting in its dominant position as a next-generation platform.
Report Scope
| Feature of the Report | Details |
| Market Size in 2025 | USD 3.66 Billion |
| Projected Market Size in 2034 | USD 5.68 Billion |
| Market Size in 2024 | USD 3.5 Billion |
| CAGR Growth Rate | 5.1% CAGR |
| Base Year | 2024 |
| Forecast Period | 2025-2034 |
| Key Segment | By Indication, Formulation, Distribution Channel and Region |
| Report Coverage | Revenue Estimation and Forecast, Company Profile, Competitive Landscape, Growth Factors and Recent Trends |
| Regional Scope |